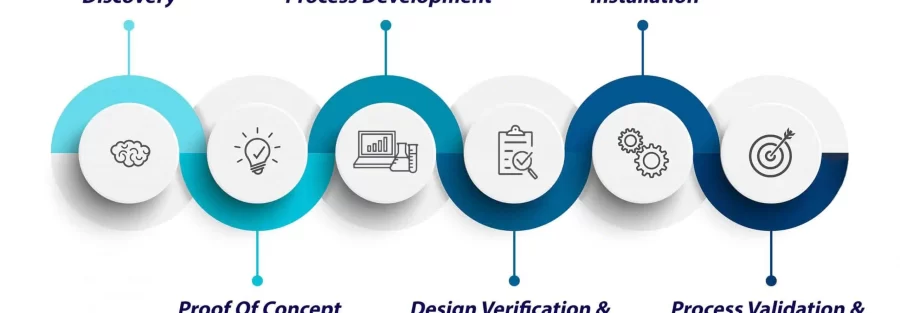
The journey of a biomedical device from conceptualization to installation is a meticulously planned and executed process, with each stage playing a crucial role in ensuring its success in a healthcare setting.
The initial phase involves ideation and conceptualization. Engineers work closely with healthcare professionals to understand specific needs and challenges, translating these insights into a design that addresses real-world scenarios. Prototyping follows, allowing for thorough testing and refinement before mass production.
Testing is a critical aspect of the design lifecycle. Rigorous evaluations ensure that the device meets regulatory standards and performs reliably in diverse conditions. The installation phase requires seamless integration into the existing healthcare infrastructure, necessitating careful planning, coordination, and user training to optimize functionality.
The lifecycle of designing and installing medical devices is a holistic process, aiming to provide healthcare professionals with tools that enhance patient care. Each step, from conceptualization to installation, contributes to the overall success of biomedical equipment in delivering safe, effective, and reliable solutions.