The seamless operation of biomedical equipment within healthcare facilities is a testament to the collaborative efforts between medical engineers and manufacturers. This behind-the-scenes partnership plays a pivotal role in ensuring the safety, reliability, and effectiveness of medical devices.
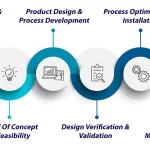
The collaborative process begins with a clear understanding of the healthcare facility’s needs and the intended use of the equipment. Manufacturers work closely with medical engineers during the design phase, incorporating specifications and features that align with industry standards and regulations. This collaboration extends to the development of prototypes, where feedback from engineers informs crucial adjustments to enhance performance.
Quality control measures are rigorously implemented throughout the manufacturing process. Thorough testing and validation protocols are established to guarantee that each biomedical device meets the highest standards before reaching healthcare facilities. Continuous communication and feedback loops between engineers and manufacturers foster an environment of ongoing improvement and innovation.
In essence, the collaboration between medical engineers and manufacturers is the unseen force driving the excellence of biomedical equipment. This synergy ensures that healthcare providers receive not just products but solutions that elevate the standards of patient care.